Cercate una Impresa Pulizie Bologna ? Ditta Eco Pulizie fornisce servizi di pulizia per privati e aziende a Bologna e Provincia. Impresa Pulizie Bologna servizi si puntano all’eccellenza e alla qualità. È abbastanza difficile trovare il tempo per pulire la tua casa, e quando devi preoccuparti di usare prodotti aggressivi che fanno male alla tua famiglia. Noi siamo qui per aiutarti.
I servizi di pulizia domestica di Eco Pulizie possono togliere il fastidio di mantenere la tua casa pulita. Offriamo una vasta gamma di servizi di pulizia personalizzabili che sono fatti su misura per soddisfare le tue esigenze e il tuo budget. Inoltre, il nostro team di professionisti garantiti e assicurati usa prodotti di alta qualità, così puoi stare tranquillo sapendo che la tua casa viene pulita in modo sicuro e sostenibile.
Offriamo servizi di pulizia della casa che usano solo prodotti e metodi ecologici, così puoi stare tranquillo sapendo che la tua casa viene pulita in modo sicuro e sano.
- Detergenti ecologici per la casa. La nostra Impresa di pulizie Bologna usa detergente biodegradabile, detersivi ecologico per la pulizia di tutta la casa. Che sia per il bagno o la cucina, per i pavimenti od ogni tipo di superficie, abbiamo la soluzione ideale. Eco Pulizie è una ditta di pulizie che vuole offrire risultati ottimali, limitando al minimo l’impatto ambientale; per questo utilizziamo prodotti con certificato CE e a marchio EcoLabel! Siamo un’ imprese di pulizie Bologna amica dell’ambiente e per questo la nostra priorità è la salute dei nostri clienti, dipendenti e per l’ambiente.
- Sanificazione Ambienti BolognaLa sanificazione dei ambienti richiede tecniche, comportamenti e tecnologie specifiche per inattivare il virus. I privati, le attività commerciali, gli enti pubblici e molti altri soggetti sono alle prese di come sanificare gli ambienti.
- Perché Impresa Pulizie Bologna Impresa di Pulizia Eco non è un’impresa pulizie Bologna come le altre. I nostri servizi di pulizia si distinguono dai nostri competitori per qualità e professionalità. Grazie all’esperienza di 5 anni maturata nel settore delle pulizie. Vi offriamo una grande gamma di servizi che vanno dalle pulizie della casa, appartamenti e ville a interventi di pulizia speciali quali post incendio, pulizie industriali e di fine cantiere. Ci trovi a Bologna e provincia.Nei suoi interventi di pulizia, Eco Pulizie ha scelto di utilizzare prodotti e materiali professionale per il rispetto dell’ambiente e la salute di clienti e dipendenti. Tra i detergenti più utilizzati nei nostri interventi professionali abbiamo: deodoranti per ambienti, detergenti per pavimenti, superfici e tappeti, sgrassanti, disincrostanti, disinfettanti, cere, lucidanti.
- Nostri addetti alle pulizie sono selezionati e assicurati fino a 500.000 euro
Nostri Servizi di Pulizia
L”Impresa di Pulizie Bologna offre servizi di Pulizia di Qualità con Sanificazione a Prezzi Accessibili. Offriamo Pulizia di Fondo, Post Trasloco, Pulizia primo Ingresso e dopo Ristrutturazione con attrezzature, detergenti professionali. Eco Pulizie è la soluzione perfetta per lei! Siamo un’impresa di pulizie domestiche professionale che si occuperà di tutto per lei, quello che deve fare è sedersi, rilassarsi e godersi la sua casa pulita.

Pulizia Appartamenti
Impresa Pulizie Bologna offre un servizio dedicato alla pulizia di appartamenti.

Pulizia Casa di Fondo
Da oltre 8 anni proponiamo servizi di pulizia profonda su misura anche per grandi ville e casali a Bologna.

Pulizia post Lavori
Servizi specializzati che eliminano ogni traccia dei lavori di ristrutturazione a Bologna e Provincia
Impresa Pulizie Bologna nasce nel 2017 ed è cresciuta incessantemente fino ad oggi!
La Pulizia Comprende ›
- Puliamo i pensili e gli interni dei mobili
- Le superfici di inox li lucidimo con INOXOL Protect
- Togliamo le griglie dei forni e li laviamo con detergente speciale
- Il forno si pulisce con gel forte Puli Forno marca Marbec
- Pulire ed igienizzare il frigorifero con il detergente Nuncas
- Sanitari, vasca, doccia eliminiamo il calcare con detergente Bad Rain
- Usiamo Active Gel WC superdisincrostante per eliminare le incrostazioni
- Igienizziamo la Box Doccia con Napisan, Vaporetto Dupray o altro prodotto adatto
- Spruzziamo con anti muffa Air MAx che previeni è rimuove la formazione di muffe
- Le mattonelle e superfici nel bagno si puliscono con detersivo professionale
- Togliamo la polvere dei Mobili con Swiffer
- Lucidiamo con prodotto per legno: MobilRain
- Spostiamo e puliamo sotto tavolini, poltrone, sedie, divani ecc.
- Rimuoviamo impronte e macchie su le superfici in legno.
- Sopra armadi aspolveriamo bene
- Puliamo dentro mobilie, casettiere, sotto il letto
- Usiamo Benhur Vetri per far splendere i vetri, tapparelle, zanzariere, infissi e inferriate
- Pulire a fondo i caloriferi, termosifoni con spugneta, specifica spazzola o vaporetto
- Il pavimento va curato e lavato per una pulizia profonda
- Si usa Detergente protettivo per pavimenti TAWIP novoSmart®
- Laviamo il pavimenti con monospazzola dopo lavori edili, costruzioni ecc.
- Spruziamo in angolini con profumo delicato Itisir fragranza Preziosa.
Impresa Pulizia Bologna
Ci adoperiamo ogni giorno allo scopo di migliorare i servizi domestiche a Bologna. Il nostro obiettivo è la vostra soddisfazione.

- Orientamento al cliente: La nostra cura nel servire il cliente è la stessa che riserveremmo a noi stessi.
- Sensibilità ambientale: I prodotti utilizzati dalla nostra Impresa Pulizie Bologna sono selezionati con cura.
- Eccellenza ed innovazione: Siamo sempre aggiornati sulle nuove tecnologie eco-compatibili. Utilizziamo prodotti e tecniche d’avanguardia.
- Crescita: Siamo consapevoli che per crescere è necessario farlo insieme. Ecco perché creiamo con i nostri collaboratori relazioni lavorative di lungo termine.
La Organizzazione
La Qualità
Perché Impresa Pulizie Bologna?
Esperienza da Oltre 4 Anni
Impresa Pulizie Bologna con team Specializzati ed una rete in continua Espansione!
Orientamento al Cliente
Siamo focalizzati sulle esigenze dei nostri Clienti e lavoriamo per la loro Soddisfazione.

Servizio Completo per Privati
forniamo un servizio di pulizia Completo per Privati con standard Ottimali.
Controllo e Qualita
Utiliziamo attrezzature moderne e prodotti con basso impatto ambientale.
Eco Pulizie: pulizie e servizi a Bologna
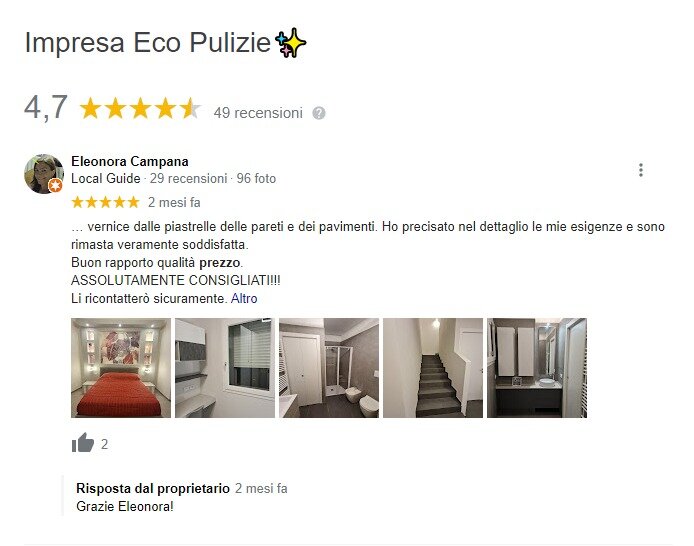
Dopo anni di lavoro ed esperienza vi possiamo fare vedere alcuni risultati dei nostri addetti. Abbiamo pulito abitazione dei privati, negozi, capannoni, scuole, appartamenti, ville, casale, vetrate ect. L’impresa di pulizie Bologna professionale Eco fornisce un ottimo servizio di pulizia di case e appartamenti. Sono in grado di affrontare qualsiasi tipo di sporco, utilizzando solo i migliori prodotti per ogni compito specifico!

Domande al Servizio di Pulizie a Bologna
Gli addetti sono assicurati in caso di danno?
Nostri addetti alle pulizie sono selezionati e assicurati fino a 500.000 euro
Per prenotare il servizio di pulizia è necessario che qualcuno visiti prima la mia abitazione?
Assolutamente no! Il preventivo sarà effettuato online in pochi minuti, seguendo le informazioni sulla tua abitazione che tu vorrai fornirci. Per noi la tua privacy è importante!
I prodotti utilizzati per la pulizia sono inclusi?
La fornitura dei prodotti per la pulizia è inclusa. Utilizzeremo prodotti ecologiche e di alta qualita.
Se non dovessi essere soddisfatto del servizio di pulizia?
Contattaci subito! Provvederemo subito a risolvere il problema. La tua soddisfazione per noi è fondamentale!
Impresa Pulizie Bologna
Impresa Pulizie Bologna fornisce servizi di pulizia a Bologna e nell’Emilia Romagna. I servizi si puntano all’eccellenza e alla qualità. Vengono coordinati dai responsabili che gestiscono le attività insieme al cliente. Si affidano i nostri servizi d’igiene ambientale uffici, centri direzionali, imprese, ditte commerciali, capannoni, case e appartamenti.

Eccellenza e qualità fanno la differenza
Ecco i vantaggi e le competenze che caratterizzano e differenziano Impresa Pulizie Bologna dai servizi standard offerti dalle ditte di pulizie bolognesi:
- Servizi di sanificazione
La impresa di pulizie Eco si occupa della disinfestazione degli ambienti pubblici e privati. - Trattamento per pavimenti
La Nostra Impresa Pulizie Bologna offre speciali trattamenti per la lucidatura di pavimenti. - Servizi per enti pubblici
I servizi di pulizia, disinfestazione e igienizzazione sono disponibili anche per aziende ed enti pubblici. - Pulizia locali commerciali
- La ditta è specializzata nella pulizia e disinfestazione di locali pubblici come le attività commerciali.
Impresa Pulizie Bologna
Impresa Pulizie a Bologna non lascia nulla caso: attenta ai dettagli conferisce ai suoi clienti un’immagine di ordine a dipendenti ed ospiti. Nello specifico definisce gli obiettivi e misura i risultati ottenuti a garanzia della qualità ed efficacia degli interventi insieme al cliente.

- Detergenti di alta qualità
A caratterizzare l’impeccabile attività d’Impresa Pulizie Bologna, vi è sicuramente l’utilizzo di prodotti di eccellente qualità, dalle attrezzature moderne fino ai detergenti di ultima gamma e gli strumenti più innovativi nel campo delle pulizie e dell’igienizzazione di ambienti. - Pulizia di casa e appartamenti
La nostra Impresa Pulizie Bologna è la ditta che cercavate per l’igienizzazione di ambienti privati e la pulizia degli casale e appartamenti a Bologna e provincia.La società di pulizie è specializzata nella pulizia di locali commerciali ed enti pubblici e propone a ogni cliente preventivi e servizi personalizzati, formulati in seguito ad attento sopralluogo. - Trattamenti di pulizia
L’agenzia di pulizie Eco propone servizi efficaci e completi per la pulizia di ambienti commerciali, industriali e residenziali, garantendo alla clientela piani personalizzati. Eco Impresa Pulizie Bologna propone preventivi su misura degli ambienti, la disinfezione e la disinfestazione, la pulizia dei da lucidatura e la levigatura di pavimenti di ogni genere e materiale. Inoltre l’azienda di pulizie interviene in provincia di Bologna per la pulizia profonda di capannoni e negozi
Per migliorare costantemente la qualità dei nostri servizi, teniamo in grande considerazione l’opinione dei nostri clienti. Pubblicitaonline.it

Cosa vi offre Impresa Pulizie Bologna?
L’impresa di pulizie Eco attiva a Bologna si rivolge a una clientela grande, composta da enti pubblici ed enti privati, fabbriche e altre aziende a cui propone un servizio completo ed efficiente di pulizia professionale degli spazi interni.
Eco Pulizie si occupa di pulizie civili e industriali, e si prende cura di appartamenti, capannoni e negozi. Inoltre, la ditta offre un servizio di pulizia di fondo da programmare alla fine dei lavori post muratori in corso.

Pulizia Domestiche Bologna
Pulire la casa è un lavoro che non piace a nessuno, ma deve essere fatto
È già abbastanza difficile trovare il tempo per pulire la tua casa, ma poi devi affrontare tutto lo sporco e la polvere che si accumulano. Per non parlare del fatto che la maggior parte dei prodotti per la pulizia sono tossici e possono essere dannosi se inalati. Pulire con Impresa Pulizie Bologna è facile e conveniente. I nostri addetti alle pulizie professionali verranno a casa tua e puliranno tutto da cima a fondo usando solo prodotti ecologici.

Esperienza Agenzia Pulizie Bologna
Con oltre sette anni di esperienza e impegno, la nostra impresa pulizie Bologna e partner più affidabile per la pulizia della casa o dell appartamento. Sempre attenti a fornire servizi professionale e di qualità, collaboriamo con ottimi risultati con privati, aziende ed enti pubblici. Abbiamo personale specializzato e sempre aggiornato.
Siamo specializzati nella sanificazione e pulizia di: Asili, Scuole, Cinema, Teatri, Appartamenti, Ville, Farmacie, Cliniche, Studi medici e veterinari, Ristoranti, Bar, Aziende Agricole, Centri Estetiche, Palestre, Negozi, Alberghi, Attività commerciali.

Azienda Pulizie Bologna
Eco Pulizie è partner di numerosi clienti sia privati sia pubblici che nella nostra azienda trovano un interlocutore professionale e affidabile. Rivolgendovi alla nostra realtà troverete un’ampia gamma di servizi di pulizia complementari che possono essere adattati con offerte mirate e personalizzate in base alle vostre esigenze.
Addetti della cui collaborazione è sempre qualificato e affidabile, costantemente aggiornato e al passo con le nuove metodologie del settore. Ciascuno dei nostri impiegati utilizza esclusivamente materiali e detergenti di alta qualità e articoli per la pulizia professionale della linea ECO.
Tutti Servizi di Pulizia
- Pulizia e Lavaggio Impianti
- Pulizia Centro Estetico, Igiene e Sanificazione
- Pulizia Cortile, Pulizie delle Aree Esterne
- Pulizia Piscine e Manutenzione della Piscina
- Pulizia Parchi, Pulizie Aree verdi Cittadine e Comunali
- Pulizie Cliniche Casa di Cura e Case di Riposo
- Pulizia Esposizioni Culturali e Musei
- Pulizia Fiere, Pulizie Stand Fiere, Pulire Stand Fieristici
- Pulizia dopo Feste, ed Eventi o Party
- Pulizia Cucine, Come Pulire le Ante e Top della Cucina
- Pulizie Straordinarie, a Fondo di Primavera Reggio Emilia
- Pulizie Straordinarie, a Fondo di Primavera Bologna
- Pulizia post Ristrutturazione Reggio Emilia
- Pulizie Straordinarie, a Fondo, Profonda di Primavera
- Pulizie post Ristrutturazione Bologna
- Pulizie di Nuove Costruzione
- Pulizia Capannoni, Depositi e Aree Industriali
- Pulizia Centri Sportivi, Palestre e Impianti Sportivi
- Pulizia Primo Ingresso, Pulizie Prima Entrata
- Come lavare e Sanificare i Materassi
- Pulizia Divani e Poltrone
- Lavaggio e Pulizia Tappeti e Moquette
- Pulizia Vetrate e Vetri
- Pulizie Ordinarie
- Pulizia Pavimenti, Pulire Cemento, Ceramica, Legno e Cotto
- Pulizie Casa di Fondo
- Pulizie Post Trasloco, Pulizia dopo Trasloco
- Pulizie Negozi e Attività Commerciali
- Pulizie di Locali e Ristoranti
- Pulizie Industriali, Cosa Sono, Quando Farle e Come si Fanno
- Pulizie Condominiali
- Pulizie Uffici
- Pulizie post Ristrutturazione
- Pulizie Casa Domestiche, Pulizia di Casa
- Pulizia Appartamenti
Consigli Pulizia
- Addetti alle Pulizie Selezionati e Assicurati
- Attrezzature moderne e prodotti professionali
- Sopralluogo, Preventivo veloce e senza impegno
- Lavare i pavimenti con macchinari
- Garantiamo qualità e professionalità

Dove Lavoriamo
L’indirizzo: Via Libia, 51, 40138 Bologna BO